1. Emission Calculation
An emissions factor is a representative value that attempts to relate the quantity of a pollutant released to the atmosphere with an activity associated with the release of that pollutant. These factors are usually expressed as the weight of the pollutant divided by unit weight, volume, distance, or duration of the activity emitting the pollutant.
AP-42, Compilation of Air Pollutant Emissions Factors from Stationary Sources, has been published since 1972 as the primary compilation of EPA's emissions factor information. It contains emissions factors and process information for over 200 air pollution source categories. A source category is a specific industry sector or group of similar emitting sources. The emissions factors have been developed and compiled from source test data, material balance studies, and engineering estimates. From EPA AP-42 Section 3.2, the formula for the Emission Factor is considered:
Cppmvd = Concentration (parts per million by volume, dry)
Qout = Stack exhaust flow rate (dry standard cubic feet per minute)
MW = Molecular weight (pounds per pound-mole)
2. Basics of Combustion
Before going further into the calculation of flue gas flow, some basis on combustion is needed. Atmospheric air is a mechanical mixture – as distinguished from a chemical reaction- of oxygen, nitrogen, and slight amounts of carbon dioxide, water vapor, argon, and other inert gases. For calculation purposes, the main components of air is assumed as oxygen and nitrogen in the ratio as given in Table 1.
| Inert Gas in Air | By Volume Percent | By Weight Percent |
|---|---|---|
| O2 | 20.91 | 23.25 |
| N2 | 79.09 | 76.85 |
The oxygen with its strong affinity for the combustible constituents of the fuel, under the proper conditions of temperature, separates itself from its mechanical union with nitrogen and enters a chemical combination with the available combustible, thus fulfilling its function in the promotion of combustion. The nitrogen serves no purpose in combustion; it absorbs heat in its passage through the combustion chamber and carries off a portion of such heat in leaving the stack.
For controls, the time taken for the burnt gas to reach the flue gas stack is very critical.
All our control calculations and algorithms are developed based on dynamic response.
But for emission, that time factor is irrelevant.
How much a plant pollutes the atmosphere with CO and NOx – a totally new perspective in measurement.
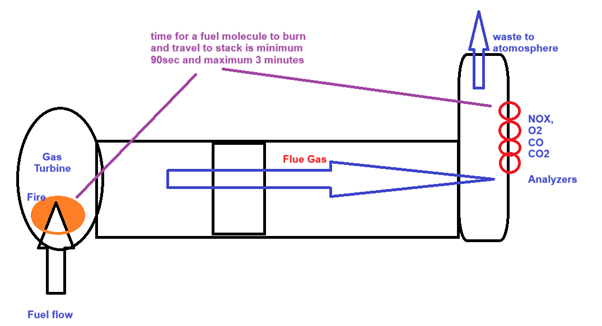
NOx represents a family of seven compounds. EPA regulates only nitrogen dioxide (NO2) as a surrogate for this family of compounds because it is the most prevalent form of NOx in the atmosphere that is generated by anthropogenic (human) activities. NO2 is not only an important air pollutant by itself but also reacts in the atmosphere to form ozone (O3) and acid rain.
It is important to note that the ozone that we want to minimize is tropospheric ozone; that is, ozone in the ambient air that we breathe. We are not talking about stratospheric ozone in the upper atmosphere that we cannot breathe. Stratospheric ozone protects us and the troposphere from ionizing radiation coming from the sun. EPA has established National Ambient Air Quality Standards (NAAQS) for NO2 and tropospheric ozone.
The NAAQS defines levels of air quality that are necessary, with a reasonable margin of safety, to protect public health (primary standard) and public welfare (secondary standard) from any known or anticipated adverse effects of pollution. The primary and secondary standard for NO2 is 0.053 parts per million (ppm) (100 micrograms per cubic meter), the annual arithmetic mean concentration.
Tropospheric ozone has been and to be a icant air pollution problem in the United States and is the primary constituent of smog. Large portions of the country do not meet the ozone NAAQS and thereby expose large segments of the population to unhealthy levels of ozone in the air. NO2 reacts in the presence of air and ultraviolet light (UV) in sunlight to form ozone and nitric oxide (NO). The NO then reacts with free radicals in the atmosphere, which are also created by the UV acting on volatile organic compounds (VOC). The free radicals then recycle NO to NO2.
In this way, each molecule of NO can produce ozone multiple times This will continue until the VOC are reduced to short chains of carbon compounds that cease to be photoreactive (a reaction caused by light). A VOC molecule can usually do this about 5 times. In addition to the NO2 and Ozone NAAQS concerns, NOx and sulfur oxides (SOx) in the atmospheres are captured by moisture to form acid rain. Acid rain, along with cloud and dry deposition, severely affects certain ecosystems and directly affects some segments of our economy.
All these facts indicate an obvious need to reduce NOx emissions. However, to successfully do so, we must understand the generation and control of the NOx family of air pollutants.
3. Stoichiometry
Stoichiometric Combustion of Methane with air can be expressed as:
Adding nitrogen to the air in the equation [2]
**From Table 1 the ratio of the volume of nitrogen to the volume of oxygen in air
4. Excess Air
In an ideal combustion, the stack will have no unburnt gas (CO). There will be no excess air, and the airflow will be stoichiometric. Based on equation [3] CO2 and N
x = number of moles of excess O2 in the excess air
Va = actual inlet airflow
Vs = stochiometric airflow
From equation [3] the stochiometric air flow is O2 + 3.768N2. From equation [4] the actual airflow is (1+x) O2 + (1+x) \times 3.768 N2. Hence equation (5) can be written as:
𝑥𝑂2 in equation [6] appears as the reaction product in equation [4]. This explains that oxygen can only appear as a reaction product if excess air is present, assuming complete combustion.
A key observation is, that the nitrogen in the flue gas came directly from the inlet combustion air, as it does not participate in combustion but carries heat (assuming the natural gas has insignificant nitrogen in composition). In other words, the actual O2 supplied in the inlet can be determined by computing the moles of O2 associated with flue gas N2.
From Table-1:
Theoretical air (stochiometric Vs) = inlet (Va) – O2 in flue gas
Applying equations (7) and (8) in equation (5)
If combustion produces both CO2 and CO, as in the case of incomplete combustion), then the Oxygen measured in the flue must be reduced by the amount of oxygen that would have combined with CO to form CO2. With CO the equation [9] can be modified as:
Equation [10] helps to calculate the excess air from the flue gas composition.
5. Example
Let us consider the natural gas composition at 42 oF as:
CO2 = 0.047%; Ethane = 1.85%; CH4 = 97.82%; N2 = 0.23%
The flue gas composition at 75 oF I given as:
NOx = 1.76 ppmvd; CO = 0.01 ppmvd; CO2 = 10.01%; O2 = 12.22%
The combined cycle fuel flow as:
Gas Turbine fuel flow: 88.14 kpph
Duct burner fuel flow: 15.72 kpph
Step:1
Calculate the total fuel flow
Conversion from KPPH to SCFH
Ideal gas law conversion factor: 379.3 SCF/lb-mole
Step:2
Calculate the stoichiometric air flow
The molecular weight of Natural Gas: 19.5 lb/mol
The molecular weight of Oxygen: 15.99 lb/mol
Total fuel flow rate: 103.86 kpph = 2020210.153 SCFH
Method I:
O2 = 32, 2O2 = 64
Therefore
Step:3
Calculate the excess air from the flue gas composition
Conversion of ppmvd to %
PPMVD stands for "parts per million by volume dry". It is a unit of measurement used in various fields, particularly in environmental science and industrial applications, to quantify the concentration of a substance in a gaseous mixture.
1 PPMVD is equivalent to 0.0001% by volume. This is because "per million" means one part in a million, and the percentage is parts per hundred.
From flue gas analysis calculates the Nitrogen in percentage volume.
Step:4
Calculate the flue gas flow
Use the results [R1] and [R2] in the equation [6]
Step:5
NOx Emission factor
Apply the value of [R3] in the equation [1] that has temperature correction.
Molecular weight of NOx = 46 lb/mol
Step:6
CO Emission factor
Apply the value of [R3] in the equation [1] that has temperature correction.
Molecular weight of CO = 28.01 lb/mol
6. Implementation
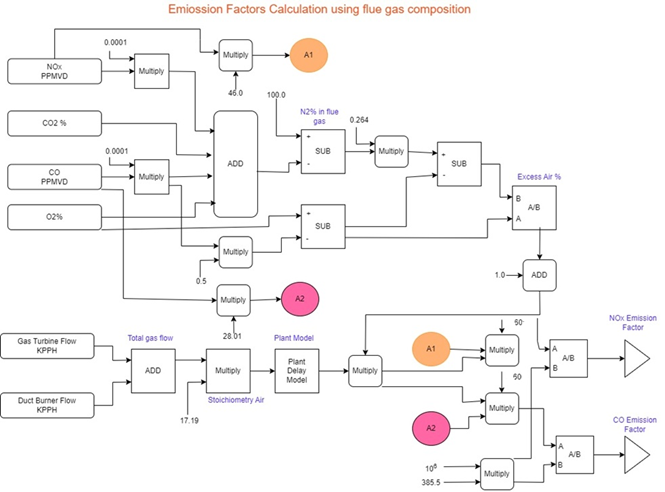
The functional block diagram is given in Figure 1. Care has to be taken to avoid being dividing by zero. More importantly, the plant model has to be provided with a delay introduced in the model for the combustion reaction.
7. Conclusion
1. Assumptions made in the plant:
- Oxygen can only appear as a reaction product if excess air is present, assuming complete combustion.
- In Emission factor calculation, Temperature Correction is not considered.
| Emission Factor/Month | VEC Data | Calculated data | ||
|---|---|---|---|---|
| Nox Lbs | Co Lbs | Nox Lbs | Co Lbs | |
| JAN 2024 | 9725 | 848 | 11942 | 1207 |
8. Sample Data
Total gas flow: 97 kpph
Stack composition:
O2 = 12.2%
CO2 = 4.9%
CO = 0.006 ppmvd
NOx = 1.103 ppmvd
8.1 Solution
1. Conversion From kpph to SCFH
Total fuel flow = 97 kpph = 97 \times 1000 = 97000 pph
Molecular weight of NOx = 46 lb/mol
Molecular weight of CO = 28.01 lb/mol
9. References
[1] Emission Factor Documentation for AP-42 Section 3.2, Natural Gas-fired Reciprocating engines, US EPA, July 2000.
[2] Combustion Fossil Power Systems, Combustion Engineering, 1981.
[3] Combustion Engineering: A reference book on fuel burning and steam generation, 1953.
[4] Principles of combustion in the steam boiler furnace. Arthur D. Pratt, The Babcock & Wilcox Co, 1919.
[5] Method 3B – Gas analysis for the determination of Emission Rate correction factor or excess air, US EPA, 2017.
[6] Compendium of Greenhouse gas emissions methodologies for the oil and natural gas industry, API – American Petroleum Institute, August 2009.

